Microorganisms rarely exist as single cells living next to each other. Instead, they accumulate along surfaces to form polymicrobial aggregates known as a film, sludge, plaque, scum, or a ‘biofilm.’ In most biofilms, the actual microorganisms account for less than 10% of the dry mass of the aggregate, whereas the biofilm matrix tends to account for over 90% of the dry weight. The biofilm matrix is the extracellular material produced by the organisms in which the microorganisms embed themselves.1 This self-produced biofilm matrix consists of extracellular polymeric substances (EPS), such as polysaccharides, extracellular DNA, proteins, lipids, and inorganic solids.2 This goopy matrix with a three-dimensional architecture protects the microorganisms lurking inside the biofilm by acting as a shield against medications, ultraviolet light, probiotics, and other substances and organisms.1,3
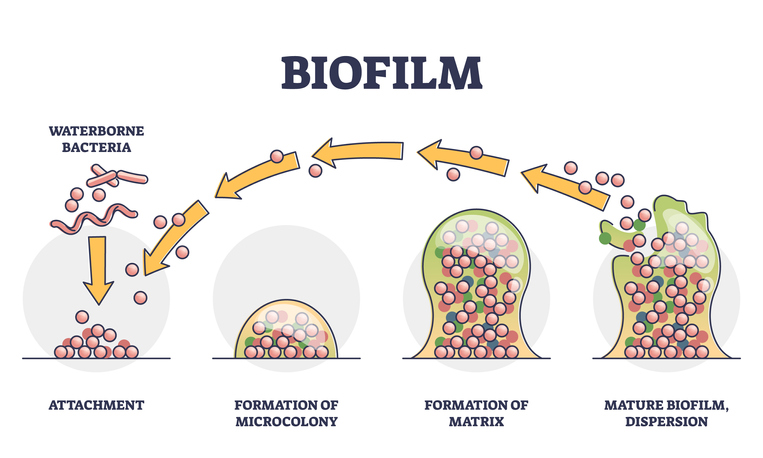
The presence of a biofilm allows a lifestyle entirely different from the lifestyle available to a single organism with no protection.1 This biofilm “lifestyle” offers numerous benefits, including enhanced nutrient uptake, limited diffusion of potentially harmful compounds, stable positioning in optimal locations, beneficial interactions between complementary neighbors, sequestration of nutrients and energy sources, temperature stabilization, and the creation of chemically stable microniches.1,2 Biofilms are common in nature and can be associated with host organisms, including humans.3 Examples of biofilms that most people are familiar with include the dental plaque on teeth and pond scum.
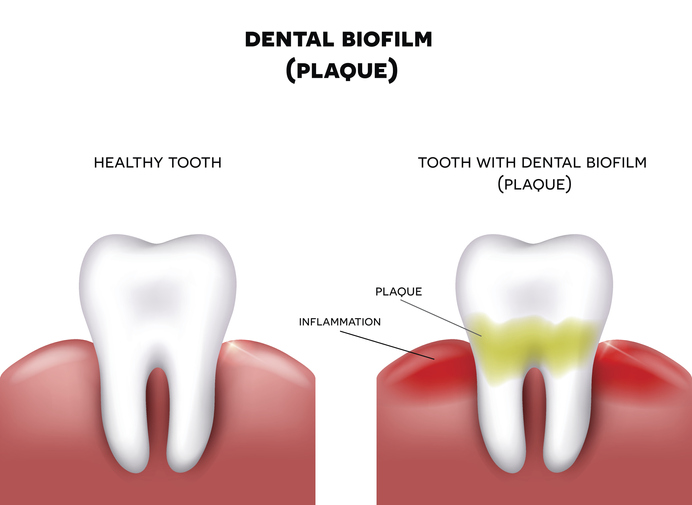
Biofilms are also an essential part of the gut microbiome since biofilms protect commensal organisms. But, more than 60% of all infections in hospitals and healthcare settings worldwide are due to bacterial pathogens that form biofilms because these pathogens have the additional layer of protection provided by the biofilm. Bacteria cohabitating within a biofilm display increased antibiotic resistance by orders of magnitude compared to those in a lonely single-cell planktonic state. Biofilms are, therefore, a significant obstacle to the treatment of many infectious diseases. Due to the protection of the biofilm, nearly 80% of biofilm-related infections do not respond to treatment with antibiotics, which leads to increased healthcare costs and patient mortality and morbidity.3
Because many pathogens reside within a biofilm community, healthcare providers now realize the optimal eradication of a pathogen often requires antimicrobials and “biofilm-busters.” Research suggests that postbiotics can act as biofilm-busters; and, therefore, could be included in a comprehensive and clinically effective treatment protocol.

When a biofilm-buster is included as part of a treatment protocol to destabilize the structure of biofilm communities, we must acknowledge that biofilm-busting can be fraught with peril. Biofilm structure destabilization and the loosening of the biofilm matrix can lead to cellular detachment of pathogenic organisms and further dissemination of biofilms, potentially leading to increasingly severe and long-term consequences. When pathogens detach from the destabilized extracellular matrix, they can then serve as inocula for new points of infection, hence leading to the spread of the infectious agent. Fortunately, postbiotics offer not only biofilm-busting properties but also antimicrobial activity against many pathogens.3
The effect of postbiotics on biofilms is evident when looking at the skin because, while bacteria thrive on our skin, biofilms are not readily apparent when the skin is not damaged or diseased. Brilliant scientists, therefore, hypothesized there must be innate mechanisms in place to inhibit or disrupt biofilm formation on the skin.4
Staphylococcus epidermidis is ubiquitous, a frequently isolated commensal present within the human skin microbiome. Previous studies have shown that Staphylococcus epidermidis has an arsenal of defense mechanisms that limit the establishment and growth of skin pathogens, including the production of postbiotic molecules that have antimicrobial activity against pathogens such as Streptococcus pyogenes and Staphylococcus aureus.4
These postbiotic antibacterial molecules are highly attractive as novel future antibiotics due to clinical efficacy, lack of harm to commensal bacteria, minimal side effects, and efficient pathogen targeting.4,5 Staphylococcus aureus can cause life-threatening infections such as pneumonia, meningitis, skin abscesses, and bloodstream infections; and, given the increasing prevalence of antibiotic-resistant Staphylococcus aureus strains, including MRSA, urgent new strategies for treatment and prevention are necessary. One potent virulence factor that renders the treatment of Staphylococcus aureus infections difficult is biofilm formation, both in the body and on non-living surfaces, including bones, heart valves, catheters, shunts, breast implants, and many other areas.4,6
One study assessed the ability of postbiotics produced by Staphylococcus epidermidis to inhibit the formation of biofilms by Staphylococcus aureus, including MRSA. When Staphylococcus aureus was grown in the presence of the Staphylococcus epidermidis postbiotic media, biofilm production was reduced by over 30% in 20 strains compared to the control with no postbiotic media. Investigators observed a more pronounced reduction of over 50% inhibition of biofilm formation for 13 of the strains, 69.2% of which were MRSA.4
The postbiotics produced by Staphylococcus epidermidis also disrupted established Staphylococcus aureus biofilms, which reduced the antibiotic concentration required to eradicate Staphylococcus aureus. Biofilm formation is one of the most powerful virulence factors unleashed by Staphylococcus aureus and is associated with chronic infections and antibiotic resistance. Therefore, postbiotics that inhibit the production of new biofilms and neutralize established biofilms could be a highly promising treatment option for Staphylococcus aureus and other infections.4
Researchers are still investigating how postbiotics affect biofilm development and stability, but we do know short-chain fatty acids (SCFAs) are postbiotic compounds that appear to play a role in biofilm management.7 Staphylococcus epidermidis produces butyric acid, which is a SCFA.8 Future research studies will likely identify additional postbiotic factors other than SCFAs that might impact the management of biofilms.

Additional evidence that postbiotics have a beneficial effect on biofilm management:
- Postbiotics produced by Lactobacillus casei target biofilm formation by the pathogen Pseudomonas aeruginosa.9
- Postbiotics produced by Lactiplantibacillus plantarum, Lactiplantibacillus curvatus EIR/DG-1, and Lactiplantibacillus curvatus EIR/BG-2 effectively reduce biofilm formation by Streptococcus mutans, which is an underlying cause of dental caries.10
- Lactobacillus paracasei postbiotic elements demonstrate antifungal activity against the biofilms produced by Candida auris.11
- Lactobacillus curvatus67 and Lactobacillus plantarum M.2 postbiotics inhibit biofilms produced by Listeria monocytogenes when applied to food contact surfaces.12
- The postbiotic short-chain fatty acids acetic acid and butyric acid can inhibit the growth of Salmonella and Shigella flexneri and affect biofilms.13
- Lactobacillus produce postbiotic substances that directly kill or inhibit the growth of, prevent attachment of, and block biofilm formation by Candida albicans.14
- Postbiotics produced by Streptococcus salivarius M18 manifest an anti-biofilm effect on Pseudomonas aeruginosa and Klebsiella pneumoniae.15
In addition to the examples above, postbiotics could be beneficial “biofilm-busters” and support the eradication of several other biofilm-producing pathogens and opportunistic pathogens, including:
Borrelia burgdorferi, Bartonella henselae, Mycoplasma spp., Klebsiella pneumoniae, Citrobacter freundii, Vibrio cholerae, Proteus mirabilis, Escherichia coli, Legionella pneumophila, Mycobacterium avium, Acinetobacter baumanii, Streptococcus pneumoniae, Haemophilus influenzae, Moraxella catarrhalis, Propionibacterium acnes, Neisseria meningitides, Prevotella spp., Porphyromonas gingivalis, Helicobacter pylori, Streptococcus pyogenes, Enterobacter spp., Candida glabrata, Candida parapsilosis, Candida dubliniensis, Candida krusei, Candida tropicalis, Pneumocystis spp., Coccidioides spp., Aspergillus spp., Cryptococcus spp., Trichosporon spp., Rhodotorula spp., Pichia spp., Geotrichum spp., Saprophytic fungi, Gardnerella vaginalis, Human T-cell Leukemia Virus Type 1 (HTLV-1), Leptospira interrogans, Burkholderia cepacia, Aeromonas hydrophila, Bacillus cereus, Bacillus pumilus, Campylobacter spp., Arcobacter-like spp., Arcobacter butzleri, Comamonas spp., Corynebacterium spp., Cronobacter sakazakii, Enterococcus spp., Hafnia alvei, Herbaspirillum spp., Kluyvera spp., Morganella morganii, Providencia rettgeri, Raoultella ornithinolytica, Serratia marcescens, Stenotrophomonas maltophilia, Yersinia enterocolitica, and others.16-48

In summary, numerous studies show that commensal bacteria secrete postbiotic products that reduce biofilm formation and remove established biofilms. Therefore, postbiotics could potentially be incorporated into an infection treatment protocol to improve clinical efficacy. There is also evidence some postbiotic compounds inhibit the replication of the pathogens while also acting as immunomodulators, though this activity could be concentration-dependent.7,13,15 To date, the interactions between postbiotics and current conventional therapies – including antibiotics – remain practically unknown.13 Future research exploring the synergistic effects of prescribing postbiotics in tandem with antibiotic, antifungal, and antiviral botanicals and medications will be fascinating.
To screen for the presence of biofilm-producing pathogens, consider ordering the Expanded Bacterial Stool Culture (GP3x) or a Comprehensive GI Health Panel that includes the GP3x. Learn more about our GI Health Panels HERE!
To place a test order, click here. As a reminder, DiagnosTechs can drop ship test kits directly to your patients. You may select this option at the top of the order form.
Please visit our Provider Tools page for more information about GI health testing.
References:
- Flemming HC, Wingender J. The biofilm matrix. Nat Rev Microbiol. 2010;8(9):623-33. doi:10.1038/nrmicro2415
- Flores-Vargas G, Bergsveinson J, Lawrence JR, et al. Environmental Biofilms as Reservoirs for Antimicrobial Resistance. Front Microbiol. 2021;12:766242. doi:10.3389/fmicb.2021.766242
- Penesyan A, Paulsen IT, Gillings MR, et al. Secondary Effects of Antibiotics on Microbial Biofilms. Front Microbiol. 2020;11:2109. doi:10.3389/fmicb.2020.02109
- Glatthardt T, Campos JCM, Chamon RC, et al. Small Molecules Produced by Commensal Staphylococcus epidermidis Disrupt Formation of Biofilms by Staphylococcus aureus. Appl Environ Microbiol. 2020;86(5):e02539-19. doi:10.1128/AEM.02539-19
- Kumar M, Sarma DK, Shubham S, et al. Futuristic Non-antibiotic Therapies to Combat Antibiotic Resistance: A Review. Front Microbiol. 2021;12:609459. doi:10.3389/fmicb.2021.609459
- Wu H, Moser C, Wang HZ, et al. Strategies for combating bacterial biofilm infections. Int J Oral Sci. 2015;7(1):1-7. doi:10.1038/ijos.2014.65
- Wilson RM, Walker JM, Yin K. Different Concentrations of Lactobacillus acidophilus Cell Free Filtrate Have Differing Anti-Biofilm and Immunomodulatory Effects. Front Cell Infect Microbiol. 2021;11:737392. doi:10.3389/fcimb.2021.737392
- Traisaeng S, Herr DR, Kao HJ, et al. A Derivative of Butyric Acid, the Fermentation Metabolite of Staphylococcus epidermidis, Inhibits the Growth of a Staphylococcus aureus Strain Isolated from Atopic Dermatitis Patients. Toxins (Basel). 2019;11(6):311. doi:10.3390/toxins11060311
- Azami S, Arefian E, Kashef N. Postbiotics of Lactobacillus casei target virulence and biofilm formation of Pseudomonas aeruginosa by modulating quorum sensing. Arch Microbiol. 2022;204(2):157. doi:10.1007/s00203-022-02770-8.
- OmerOglou E, Karaca B, Kibar H, et al. The role of microbiota-derived postbiotic mediators on biofilm formation and quorum sensing-mediated virulence of Streptococcus mutans: A perspective on preventing dental caries. Microb Pathog. 2022;164:105390. doi:10.1016/j.micpath.2022.105390
- Rossoni RD, de Barros PP, Mendonça IDC, et al. The Postbiotic Activity of Lactobacillus paracasei4 Against Candida auris. Front Cell Infect Microbiol. 2020;10:397. doi:10.3389/fcimb.2020.00397
- Hossain MI, Mizan MFR, Roy PK, et al. Listeria monocytogenes biofilm inhibition on food contact surfaces by application of postbiotics from Lactobacillus curvatus67 and Lactobacillus plantarum M.2. Food Res Int. 2021;148:110595. doi:10.1016/j.foodres.2021.110595
- Huang CB, Alimova Y, Myers TM, et al. Short- and medium-chain fatty acids exhibit antimicrobial activity for oral microorganisms. Arch Oral Biol. 2011;56(7):650-654. doi:10.1016/j.archoralbio.2011.01.011
- Vazquez-Munoz R, Dongari-Bagtzoglou A. Anticandidal Activities by Lactobacillus Species: An Update on Mechanisms of Action. Front Oral Health. 2021;2:689382. doi:10.3389/froh.2021.689382
- Tunçer S, Karaçam S. Cell-free supernatant of Streptococcus salivarius M18 impairs the pathogenic properties of Pseudomonas aeruginosa and Klebsiella pneumonia. Arch Microbiol. 2020;202(10):2825-2840. doi:10.1007/s00203-020-02005-8
- Sapi E, Gupta K, Wawrzeniak K, et al. Borrelia and Chlamydia Can Form Mixed Biofilms in Infected Human Skin Tissues. Eur J Microbiol Immunol (Bp). 2019;9(2):46-55. doi:10.1556/1886.2019.00003
- Okaro U, Green R, Mohapatra S, et al. The trimeric autotransporter adhesin BadA is required for in vitro biofilm formation by Bartonella henselae. NPJ Biofilms Microbiomes. 2019;5(1):10. doi:10.1038/s41522-019-0083-8
- Yaita K, Gotoh K, Nakano R, et al. Biofilm-Forming by Carbapenem Resistant Enterobacteriaceae May Contribute to the Blood Stream Infection. Int J Mol Sci. 2019;20(23):5954. doi:10.3390/ijms20235954
- Schulze A, Mitterer F, Pombo JP, et al. Biofilms by bacterial human pathogens: Clinical relevance – development, composition and regulation – therapeutical strategies. Microb Cell. 2021;8(2):28-56. doi:10.15698/mic2021.02.741
- Ramage G, Rajendran R, Sherry L, et al. Fungal biofilm resistance. Int J Microbiol. 2012;2012:528521. doi:10.1155/2012/528521
- Gharaghani M, Taghipour S, Zarei Mahmoudabadi A. Molecular identification, biofilm formation and antifungal susceptibility of Rhodotorula Mol Biol Rep. 2020;47(11):8903-8909. doi:10.1007/s11033-020-05942-1
- Perpetuini G, Rossetti AP, Battistelli N, et al. Adhesion Properties, Biofilm Forming Potential, and Susceptibility to Disinfectants of Contaminant Wine Yeasts. Microorganisms. 2021;9(3):654. doi:10.3390/microorganisms9030654
- Fröhlich-Wyder MT, Arias-Roth E, Jakob E. Cheese yeasts. 2019;36(3):129-141. doi:10.1002/yea.3368
- Ristow P, Bourhy P, Kerneis S, et al. Biofilm formation by saprophytic and pathogenic leptospires. Microbiology (Reading). 2008;154(Pt 5):1309-1317. doi:10.1099/mic.0.2007/014746-0
- Filardo S, Di Pietro M, Tranquilli G, et al. Biofilm in Genital Ecosystem: A Potential Risk Factor for Chlamydia trachomatis Infection. Can J Infect Dis Med Microbiol. 2019;2019:1672109. doi:10.1155/2019/1672109
- Awadh AA, Le Gresley A, Forster-Wilkins G, et al. Determination of metabolic activity in planktonic and biofilm cells of Mycoplasma fermentans and Mycoplasma pneumoniae by nuclear magnetic resonance. Sci Rep. 2021;11(1):5650. doi:10.1038/s41598-021-84326-2
- Thoulouze MI, Alcover A. Can viruses form biofilms? Trends Microbiol. 2011;19(6):257-62. doi:10.1016/j.tim.2011.03.002
- Maali Y, Journo C, Mahieux R, et al. Microbial Biofilms: Human T-cell Leukemia Virus Type 1 First in Line for Viral Biofilm but Far Behind Bacterial Biofilms. Front Microbiol. 2020;11:2041. doi:10.3389/fmicb.2020.02041
- Thibeaux R, Soupé-Gilbert ME, Kainiu M, et al. The zoonotic pathogen Leptospira interrogans mitigates environmental stress through cyclic-di-GMP-controlled biofilm production. NPJ Biofilms Microbiomes. 2020;6(1):24. doi:10.1038/s41522-020-0134-1
- Teitzel GM, Parsek MR. Heavy metal resistance of biofilm and planktonic Pseudomonas aeruginosa. Appl Environ Microbiol. 2003;69(4):2313-2320. doi:10.1128/AEM.69.4.2313-2320.2003
- Brackman G, Coenye T. Quorum sensing inhibitors as anti-biofilm agents. Curr Pharm Des. 2015;21(1):5-11. doi:10.2174/1381612820666140905114627
- Lee N, Kim MD, Lim MC. Autoinducer-2 Could Affect Biofilm Formation by Food-Derived Bacillus cereus. Indian J Microbiol. 2021;61(1):66-73. doi:10.1007/s12088-020-00918-y
- Zhu ML, Wang YH, Dai Y, et al. Effects of Different Culture Conditions on the Biofilm Formation of Bacillus pumilus HR10. Curr Microbiol. 2020;77(8):1405-1411. doi:10.1007/s00284-020-01944-1
- Šilha D, Sirotková S, Švarcová K, et al. Biofilm Formation Ability of Arcobacter-like and Campylobacter Strains under Different Conditions and on Food Processing Materials. Microorganisms. 2021;9(10):2017. doi:10.3390/microorganisms9102017
- Girbau C, Martinez-Malaxetxebarria I, Muruaga G, et al. Study of Biofilm Formation Ability of Foodborne Arcobacter butzleri under Different Conditions. J Food Prot. 2017:758-762. doi:10.4315/0362-028X.JFP-16-505
- Wang Y, Huang Z, Zhou N, et al. The Response Regulator FlmD Regulates Biofilm Formation in Comamonas testosteroni through the Transcriptional Activator SoxR. Microorganisms. 2022;10(2):356. doi:10.3390/microorganisms10020356
- Ozdemir S, Aydogan O, Koksal Cakirlar F. Biofilm Formation and Antimicrobial Susceptibility of Non-Diphtheria Corynebacterium Strains Isolated from Blood Cultures: First Report from Turkey. Medeni Med J. 2021;36(2):123-129. doi:10.5222/MMJ.2021.60252
- Henry M, Fouladkhah A. Outbreak History, Biofilm Formation, and Preventive Measures for Control of Cronobacter sakazakii in Infant Formula and Infant Care Settings. Microorganisms. 2019;7(3):77. doi:10.3390/microorganisms7030077
- Ch’ng JH, Chong KKL, Lam LN, et al. Biofilm-associated infection by enterococci. Nat Rev Microbiol. 2019;17(2):82-94. doi:10.1038/s41579-018-0107-z
- Pang Y, Wang S, Tao J, et al. Mechanism of berberine hydrochloride interfering with biofilm formation of Hafnia alvei. Arch Microbiol. 2022;204(2):126. doi:10.1007/s00203-021-02617-8
- Macedo AJ, Kuhlicke U, Neu TR, et al. Three stages of a biofilm community developing at the liquid-liquid interface between polychlorinated biphenyls and water. Appl Environ Microbiol. 2005;71(11):7301-7309. doi:10.1128/AEM.71.11.7301-7309.2005
- Røder HL, Hansen LH, Sørensen SJ, et al. The impact of the conjugative IncP-1 plasmid pKJK5 on multispecies biofilm formation is dependent on the plasmid host. FEMS Microbiol Lett. 2013;344(2):186-92. doi:10.1111/1574-6968.12175
- Guzman JPMD, De Las Alas TPL, Lucban MC, et al. Green tea (Camellia sinensis) extract inhibits biofilm formation in acyl homoserine lactone-producing, antibiotic-resistant Morganella morganii isolated from Pasig River, Philippines. Heliyon. 2020;6(10):e05284. doi:10.1016/j.heliyon.2020.e05284
- Sagar S, Narasimhaswamy N, D’Souza J. Providencia Rettgeri: An Emerging Nosocomial Uropathogen in an Indwelling Urinary Catheterised Patient. J Clin Diagn Res. 2017;11(6):DD01-DD02. doi:10.7860/JCDR/2017/25740.10026
- Büyükcam A, Liste Ü, Bıçakçıgil A, et al. A case of Raoultella ornithinolytica urinary tract infection in a pediatric patient. J Infect Chemother. 2019;25(6):467-469. doi:10.1016/j.jiac.2018.12.011
- Fu Y, Liu W, Liu M, et al. In vitro anti-biofilm efficacy of sanguinarine against carbapenem-resistant Serratia marcescens. 2021;37(3):341-351. doi:10.1080/08927014.2021.1919649
- Flores-Treviño S, Bocanegra-Ibarias P, Camacho-Ortiz A, et al. Stenotrophomonas maltophilia biofilm: its role in infectious diseases. Expert Rev Anti Infect Ther. 2019;17(11):877-893. doi:10.1080/14787210.2019.1685875
- Lenchenko E, Lozovoy D, Strizhakov A, et al. Features of formation of Yersinia enterocolitica biofilms. Vet World. 2019;12(1):136-140. doi:10.14202/vetworld.2019.136-140

