
 Toxoplasmosis – Symptoms, Diagnosis & Treatment
Toxoplasmosis – Symptoms, Diagnosis & Treatment
Toxoplasmosis is caused by the protozoan parasite Toxoplasma gondii. T. gondii currently infects approximately one-third of the global population. After infection, T. gondii forms cysts in tissues, including the brain, which can persist in the human body for life.1
Toxoplasmosis is most concerning during pregnancy and in immunocompromised patients. In immunocompetent individuals, toxoplasmosis is primarily considered a benign condition. However, acute toxoplasmosis in individuals with healthy immune systems can cause severe sequelae, including myocarditis, pericarditis, heart failure, pneumonia, respiratory failure, encephalitis, meningitis, Guillain-Barré syndrome, and death.2,3 Furthermore, the latest research suggests that even latent or subclinical toxoplasmosis can cause significant chronic health concerns in otherwise healthy and immunocompetent patients.4,5
 What is Toxoplasmosis?
What is Toxoplasmosis?
Toxoplasmosis is a Toxoplasma gondii infection. There are several clinically distinct forms of toxoplasmosis, including:
- Acute toxoplasmosis6
- Cerebral toxoplasmosis7
- Ocular toxoplasmosis8
- Pulmonary toxoplasmosis9
- Chronic toxoplasmosis5
- Congenital toxoplasmosis10,11
- Latent (subclinical) toxoplasmosis5,12
Congenital toxoplasmosis is characterized by the vertical transmission of T. gondii from mother to fetus.10 Acute toxoplasmosis in immunocompetent individuals presents as a flu-like illness characterized by nonspecific constitutional symptoms. Since T. gondii is neurotropic and oculotropic, it can also cause acute cerebral toxoplasmosis and ocular toxoplasmosis.10
Pulmonary toxoplasmosis occurs when T. gondii infects the lungs. Pulmonary toxoplasmosis can be challenging to diagnose because it is rare and has a variable clinical presentation. A review published in 2007 discovered only nine cases of pulmonary toxoplasmosis in the medical literature. Of note, most patients were young men, with a median age of 34 years, who had consumed raw or undercooked meat.13
Chronic toxoplasmosis is a long-term infection with persistent or recurrent clinical symptoms that may require medical treatment. Latent toxoplasmosis is a clinically mild, long-term (often lifelong) “asymptomatic” infection that may present with subtle, nonspecific symptoms or behavioral changes, some of which may worsen over time.5 Latent toxoplasmosis may progress to a chronic form, and chronic toxoplasmosis may revert to a latent subclinical state. Latent toxoplasmosis can reactivate into an acute infection with immunocompromise. Environmental factors, including stress, other infections, and certain medications, may also trigger reactivation.5
 The Life Cycle of Toxoplasma gondii – Toxoplasmosis in Cats and Humans
The Life Cycle of Toxoplasma gondii – Toxoplasmosis in Cats and Humans
Toxoplasma gondii only sexually reproduces in cats (domestic and wild); therefore, cats are the definitive host and ultimate source of all infections. Despite not sexually reproducing in humans, T. gondii does infect humans, leading to toxoplasmosis.14 Therefore, humans serve as intermediate hosts. Many other animals, including mice and birds (eaten by cats), also serve as intermediate hosts.15
There are four forms of Toxoplasma gondii:
- Oocysts, which are shed in the feces of the definitive feline host.
- Oocysts contain sporozoites and become infectious after sporulation.
- Tachyzoites – rapidly multiplying organisms found in the tissues.
- Bradyzoites – slowly multiplying organisms found in the tissues.
- Tissue cysts – walled structures that contain bradyzoites and are resistant to the immune system.15
Infected cats excrete between 2 and 20 million oocysts per day in their feces.16 After excretion, the oocysts sporulate in the environment until they produce eight haploid sporozoites encased within the oocyst wall. Sporulation occurs in 1 to 5 days under ideal conditions, but could take several weeks.15,16 The oocysts are not infectious until after sporulation; therefore, changing cat litter daily can help prevent transmission.17
Most cats excrete oocysts for 1-2 weeks; however, some cats can continue shedding for up to 3-4 weeks after infection. T. gondii oocysts are stable for over a year under a variety of environmental conditions.15,16
 When T. gondii is ingested by an intermediate host, such as a human, the tissue cyst or oocyst wall is dissolved during digestion, which releases the bradyzoites or sporozoites into the gut lumen. The bradyzoites or sporozoites then enter the lamina propria of the small intestine and begin to multiply as tachyzoites. The tachyzoites disseminate to extraintestinal tissues within a few hours of infection via lymphatic and blood circulation.15
When T. gondii is ingested by an intermediate host, such as a human, the tissue cyst or oocyst wall is dissolved during digestion, which releases the bradyzoites or sporozoites into the gut lumen. The bradyzoites or sporozoites then enter the lamina propria of the small intestine and begin to multiply as tachyzoites. The tachyzoites disseminate to extraintestinal tissues within a few hours of infection via lymphatic and blood circulation.15
Tachyzoites can enter nearly any cell and multiply until the host cell ruptures. The released tachyzoites from the ruptured cell then enter surrounding cells. As the immune system begins to respond, the tachyzoites will disappear and form bradyzoites within tissue cysts to escape the immune response.1,15
Tissue cysts can form in many organs, but are most commonly found in the skeletal muscles, myocardium, and the central nervous system (CNS) in humans. Unfortunately, the tissue cysts with bradyzoites generally do not cause a host reaction and can persist for many years, possibly for life.15
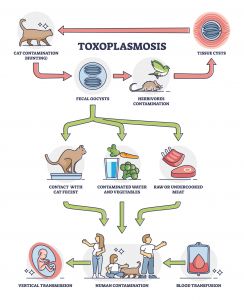 Toxoplasma gondii is transmitted to humans via four routes:
Toxoplasma gondii is transmitted to humans via four routes:
- Ingestion of infectious oocysts from the environment, via contaminated water, hands, soil, fruits, or vegetables.
- Ingestion of undercooked meat that contains tissue cysts.
- Transmission from an infected mother to the fetus.
- Transplantation of an infected organ from an infected donor.15
Some research suggests that T. gondii can be transmitted sexually from male to female, which could increase the risk of congenital toxoplasmosis. The potential for the sexual transmission of Toxoplasma requires further study.12,18,19 Research also indicates that ticks could be a reservoir for the transmission of Toxoplasma; however, more investigation is needed.15,20-22
 Toxoplasmosis – Signs and Symptoms
Toxoplasmosis – Signs and Symptoms
The severity of toxoplasmosis ranges from benign and asymptomatic to life-threatening with severe multi-organ involvement.1
The most common signs and symptoms of acute toxoplasmosis in immunocompetent individuals include:
- Chills10
- Elevated Toxoplasma gondii-specific antibody levels9
- Fever23
- Fatigue24
- Headaches10
- Hepatosplenomegaly24
- Lymphadenopathy10,24
- Myalgias10
- Pharyngitis24
- Rash10
 Acute toxoplasmosis can be mistaken for the flu or an infection with the pathogens that cause mononucleosis, such as EBV or CMV. 24,25 In immunocompetent individuals, infection with Toxoplasma gondii is considered self-limited.26 However, the latest research shows that acute and latent toxoplasmosis in individuals with healthy immune systems can cause severe repercussions.2,10
Acute toxoplasmosis can be mistaken for the flu or an infection with the pathogens that cause mononucleosis, such as EBV or CMV. 24,25 In immunocompetent individuals, infection with Toxoplasma gondii is considered self-limited.26 However, the latest research shows that acute and latent toxoplasmosis in individuals with healthy immune systems can cause severe repercussions.2,10
Other signs, symptoms, sequelae, and health concerns associated with toxoplasmosis include:
Research suggests that Toxoplasma gondii may alter neurotransmitter levels and genetic expression, leading to many of the health concerns listed above.28,84,85 During its life cycle, research shows that T. gondii interacts with approximately 3000 host genes or proteins.28 T. gondii also influences the specific brain regions for cognition, mood, and emotion processing, neuroinflammation, and neuroendocrine changes to contribute to seemingly unrelated health concerns.49
While the bradyzoites in tissue cysts have traditionally been viewed as latent or “resting,” new research suggests that they continue to replicate. Therefore, tissue cysts can occasionally rupture and release parasites, which are readily controlled by the immune response in immunocompetent individuals. However, they can multiply and quickly disseminate if the host is immunocompromised.15
Acute toxoplasmosis is exceptionally severe in immunocompromised patients. Cases of disseminated toxoplasmosis after a stem cell transplant can reach a mortality rate of 80% with a median survival time of only 10 days post-diagnosis.51
 Testing for Toxoplasmosis
Testing for Toxoplasmosis
Ideally, patients with any of the health concerns noted above will be tested for toxoplasmosis.10 Also, consider screening for asymptomatic latent or subclinical toxoplasmosis in those who are at increased risk of exposure to Toxoplasma gondii, including your patients who work in veterinary medicine.86
Diagnosing toxoplasmosis can be challenging in immunocompetent and immunocompromised patients. The diagnosis is easily missed in immunocompetent patients due to a lack of clinical suspicion. Toxoplasmosis often presents with nonspecific symptoms that mimic mononucleosis or the flu in this patient population.10 In immunocompetent individuals, serological testing for IgM and IgG antibodies in the blood is the diagnostic standard.10,24
Research suggests that IgM antibodies are detectable 5 days after infection and reach maximum levels in 1 to 2 months. IgG antibodies are detectable 1 to 2 weeks after infection and reach the highest levels in 3 to 6 months. Since they mediate long-term immunity, IgG antibodies are present in a significant percentage of the general population and often remain detectable for life in those with latent toxoplasmosis. A lack of IgM antibodies coupled with a high IgG level contributes to the diagnostic challenge.10
Assessing the secretory IgA (sIgA) antibody level in saliva can be ordered as a screening test or a valuable addition to the standard diagnostic work-up. Some evidence suggests that elevated IgA (including secretory IgA) levels may correlate well with an acute infection. Researchers, however, have also found elevated sIgA levels during latent toxoplasmosis. All positive findings, including an elevated T. gondii-specific salivary sIgA antibody level, must be interpreted in the context of the complete clinical picture and may require further assessment.87-95
In immunosuppressed patients, serologic testing is often not clinically useful due to an insufficient immune response and a lack of antibodies. Imaging, such as computed tomography (CT), positron emission tomography (PET), or magnetic resonance imaging (MRI), can support the diagnosis of toxoplasmosis in this patient population.10
While a standardized PCR test for toxoplasmosis is not available, PCR testing can diagnose toxoplasmosis in all patient populations. T. gondii DNA can be detected in blood and other bodily fluids, including cerebrospinal fluid and the aqueous humor of the eye. The detection of Toxoplasma DNA provides a definitive diagnosis.10,24,96
Muscle, lymph node, or organ biopsy also provides a definitive diagnosis based on the presence of tissue cysts, but it is a highly invasive procedure. A biopsy is rarely performed and often only ordered for patients who fail to show clinical or radiological improvement of symptoms within 14 days of treatment initiation.10,14 Amniocentesis and ultrasound can be ordered to diagnose toxoplasmosis during pregnancy.24 Additional tests that diagnose pulmonary toxoplasmosis include bronchoalveolar lavage and sputum analysis.68
 Toxoplasmosis Prevention
Toxoplasmosis Prevention
Since the diagnosis of toxoplasmosis can be challenging and an infection with Toxoplasma gondii may result in lifelong or life-threatening health concerns, the prevention of T. gondii transmission is crucial.
Reduce the risk of a Toxoplasma gondii infection with the following guidelines:
- Avoid adopting stray cats and kittens. Younger outdoor cats are more likely to release Toxoplasma oocysts in their feces.17
- Avoid eating raw oysters, clams, and mussels.24,45
- Avoid unpasteurized milk, including goat milk.10,24
- Wear gloves when touching cat litter boxes, gardening, or handling any sand or soil that may have been in contact with cat feces. Also, perform appropriate hand hygiene afterward.10,24,45
- Change cat litter boxes daily, unless pregnant or immunocompromised, in which case someone else must change the litter box daily.17
- Cook all foods to safe temperatures.10,45
- Do not feed cats undercooked or raw meat and keep them indoors.24
- Drink only treated, boiled, or well-filtered water.24,45
- Freeze all meats for several days at sub-zero temperatures before cooking.10
- Wash or peel all fruits and vegetables.10,24,45
- Wash all surfaces, including utensils, dishes, cutting boards, and counters, that have come in contact with raw meat, poultry, seafood, and unwashed fruits or vegetables.10,17,24
- Cover outdoor children’s sandboxes to prevent cats from using them as a litter box.97
 Naturopathic Toxoplasmosis Treatment & Management
Naturopathic Toxoplasmosis Treatment & Management
According to the Mayo Clinic, toxoplasmosis is often benign and does not require treatment in immunocompetent patients.98 However, as explained above, even latent toxoplasmosis might cause long-term sequelae. All currently available therapeutic pharmaceutical regimens are only effective against the tachyzoites and cannot eliminate the long-term tissue cysts. Fortunately, early evidence suggests that some naturopathic treatment options may reduce the risk of infection and interfere with cyst development.49
Myristica fragrans (Nutmeg)
Myristica fragrans (nutmeg) is a spice used in foods and folk medicine. The bioactive constituent myrislignan perturbs the tachyzoite’s mitochondrial function, and pre-clinical research suggests that M. fragrans is also effective against the bradyzoite stage. In an animal study, M. fragrans offered minor protection against brain cyst development.49
Nigella sativa (Black Cumin)
Nigella sativa (black cumin) has a long history of use in traditional medicine. Modern clinical trials demonstrate that N. sativa is safe and effective for the treatment of autoimmune, inflammatory, and metabolic disorders. Animal studies show that the volatile oil component of N. sativa acts synergistically with the pharmaceutical pyrimethamine to reduce T. gondii load and damage to the liver and spleen during an acute infection. Preclinical evidence demonstrates that N. sativa seed oil may offer prophylactic and therapeutic effects during a chronic infection. N. sativa seed oil administered to animal models before infection or a few days after infection significantly reduced mortality and brain cyst load. The treated groups also exhibited an enhanced level of inducible nitric oxide synthase (iNOS) and fewer histopathological alterations compared with the untreated group.49
 Thymus spp. (Thyme) Essential Oil
Thymus spp. (Thyme) Essential Oil
Administering Thymus spp. (thyme) essential oil at the time of or shortly after infection prevents the formation of brain cysts, according to an animal study. Administration of thyme extract to chronically infected animal models also lowers the brain cyst load and ameliorates the inflammatory brain lesions.49
Curcuma longa (Turmeric)
Curcuma longa (turmeric) is safe and effective for treating many diseases, based on the results of extensive clinical trials. One of the bioactive constituents in turmeric is curcumin, a pleiotropic compound that targets multiple signaling pathways. The FDA designates curcumin as a Generally Recognized as Safe (GRAS) dietary compound. Research shows that curcumin blocks parasite growth by inhibiting T. gondii glyoxalase 1 (GLO1) activity and targets the T. gondii detoxification pathway. However, the clinical bioactivity of curcumin is often limited by its low oral bioavailability, which is why researchers have developed nanocurcumin. Nanocurcumin encapsulates curcumin in nanomaterials to improve the solubility and therapeutic applications of curcumin, including as antiparasitic agents.49
In an animal model of acute toxoplasmosis, curcumin and nanocurcumin improved survival and reduced peritoneal tachyzoite load in infected animals. Moreover, when curcumin and the nanocurcumin were administered shortly after infection, significant reductions in the size and number of brain cysts in chronically infected animal models were noted. When administered separately, the nanocurcumin was superior to regular curcumin in alleviating the brain cyst burden.49
Resveratrol
Like curcumin, resveratrol is a polyphenol that also targets multiple cellular signaling pathways. Numerous clinical trials have demonstrated the efficacy, safety, pharmacokinetics, and promising therapeutic effects of resveratrol against cardiovascular, neurological, and metabolic diseases. To enhance the bioavailability and bioactivity, nanotechnology formulations of resveratrol have been developed.49
Research shows that pairing resveratrol nanoparticles with the pharmaceutical sulfamethoxazole-trimethoprim (ST) alleviates brain cyst burden, decreases the levels of serum pro-inflammatory cytokines, and increases the level of interleukin-10. The combined resveratrol & ST regimen also mitigates the cognitive and behavioral impairments normally observed during a chronic infection in the central nervous system (CNS). Unfortunately, the administration of free resveratrol, the nanoparticles, or ST alone showed no effect on the neurological sequelae; the benefits are truly synergistic.49 Outside of the CNS, evidence from pre-clinical studies suggests that resveratrol may modulate or inhibit damage in some organs, including the liver and lungs.99-102
 Ellagitannins & Urolithin-A (UA) from Pomegranate
Ellagitannins & Urolithin-A (UA) from Pomegranate
Pomegranate is a rich source of the polyphenols known as ellagitannins. Ellagitannins are metabolized to urolithins by the human gut microbiome. Urolithin-A (UA) is a powerful neuroprotectant against neurodegeneration, ischemic neuronal injury, and brain aging. Furthermore, in a human clinical trial, UA demonstrated a favorable safety profile and improved muscle health in elderly individuals. Pomegranate and UA also exhibit anti-T. gondii activity. UA interferes with intracellular tachyzoite growth and cyst formation in infected neural cells. In an animal study, the daily injection of UA shortly after infection reduced the severity of a chronic CNS infection compared to untreated animals.49
Rosmarinus officinalis (Rosemary)
Rosmarinus officinalis (Rosemary) extracts perturb cyst wall integrity. The administration of alcoholic and oil extracts 1 week before infection or 2 weeks following infection with T. gondii significantly reduced the brain cyst burden, cyst viability, and histopathological insults compared with the untreated group in an animal study. The surfaces of the cysts were marked with multiple depressions, protrusions, and irregularities in the brains of the treated animals upon ultrastructural analysis of the cysts via scanning electron microscopy. Furthermore, the isolation and inoculation of the damaged cysts into a healthy animal did not produce an infection, highlighting a loss in cyst viability after the administration of Rosemary in animal models.49
 Artemisinin and Derivatives from Artemisia annua
Artemisinin and Derivatives from Artemisia annua
Artemisinin (ART) is the bioactive constituent of Artemisia annua. ART and its derivatives, including artesunate, artemether, artemiside, and artemisone, have been reported to exhibit activity against tachyzoite growth in vitro that could control acute infection and prevent reactivated toxoplasmosis. ART appears to control tachyzoite growth by interfering with the parasite’s calcium homeostasis and mitochondrial physiology. In an animal study, the co-administration of artesunate and dihydroartemisinin interfered with the progression of chronic toxoplasmosis by perturbing cyst wall integrity. However, the cysts remained viable and produced secondary infections in healthy animals.49
Essential Omega-3 Polyunsaturated Fatty Acids
Abundant omega-3 polyunsaturated fatty acids could be protective against toxoplasmosis. One animal study showed that fish oil supplementation prevented toxoplasmosis. Researchers suspect that the fish oil disturbs fatty acid metabolism and availability for T. gondii growth. In another animal study, chronically infected animals with elevated levels of omega-3 polyunsaturated fatty acids exhibited lower brain cyst burdens compared to the animals with normal fatty acid levels.49
The Flavonoid Quercetin
Quercetin is a ubiquitous flavonoid present in many fruits and vegetables. Evidence suggests that quercetin may suppress tachyzoite differentiation into bradyzoites by inhibiting T. gondii Heat Shock Protein 70 (HSP70) and HSP90 synthesis. Since quercetin can cross the blood-brain barrier, it is plausible that quercetin could influence brain cyst burden and offer potential therapeutic value against chronic toxoplasmosis in the brain.49
 Melatonin & Zinc for Immune Support
Melatonin & Zinc for Immune Support
The results of one animal study suggest that supplementation with melatonin and zinc could be considered as an adjunctive therapy to the classic treatment of Toxoplasma retinochoroiditis if the beneficial outcomes can be confirmed in a clinical setting. Neither melatonin nor zinc alone demonstrated benefit. The combination of zinc and melatonin increased the infiltration of lymphocytes, CD3+, CD4+, and CD8+ cells as part of the immune response against T. gondii.103
Another study analyzed the effects of melatonin in monkey kidney cell epithelial cells after infection with T. gondii. Melatonin treatment reduced parasite proliferation in the kidney cells. After treatment with melatonin, T. gondii appeared to experience an apoptotic-like cell death. The results suggest that the use of melatonin or melatonin derivatives could be considered as an alternative or adjunctive treatment for toxoplasmosis in the future after further investigation.104
Lentinula edodes (Shiitake Mushroom)
Finally, the results of one animal study suggest that the bioactive constituent lentinan from Lentinula edodes (Shiitake mushroom) may protect against toxoplasmosis. While lentinan did not directly inhibit parasite growth in the study, lentinan prevented T. gondii-induced cognitive deficits and decreased the cyst burden, which correlated with behavioral performance. Lentinan also altered the transcriptome profile of genes related to neuroinflammation, microglial activation, synaptic function, neural development, and cognitive behavior in the hippocampus of infected animals. Moreover, lentinan reduced the infection-induced accumulation of microglia, ameliorated the neurite and synaptic ultrastructural damage, and downregulated the mRNA expression of proinflammatory cytokines.105
Further clinical research is required to confirm the protective effects of curcumin, quercetin, artesunate, resveratrol, melatonin, lentinan, and other naturopathic treatments for toxoplasmosis in humans. Unfortunately, the absence of clinically appropriate biomarkers for measuring brain cyst burden is a significant impediment to assessing the effectiveness of treatment options in humans. Neuroimaging techniques, such as MRI and CT, can assess the severity of toxoplasmic encephalitis. However, they cannot quantify the brain cyst burden. The lack of non-invasive techniques to monitor bradyzoite and cyst development in the brain makes clinical research difficult, especially when monitoring disease progression in immunocompetent patients with subclinical, latent, or chronic toxoplasmosis.49
Additional naturopathic and pharmaceutical treatment options are offered in your Provider Portal in the document: Toxoplasmosis Treatment Protocols (PDF)
 Test for Parasites with DiagnosTechs Laboratory
Test for Parasites with DiagnosTechs Laboratory
DiagnosTechs is known as the laboratory that detects the parasites that other laboratories may miss.
Screen for toxoplasmosis with our non-invasive Toxoplasma gondii antibody, sIgA saliva test, which is available as an individual test or included with these test panels:
To place a test order, click here. DiagnosTechs will drop ship test kits directly to your patients. You may select this option at the top of the order form.
 References:
References:
- Fabiani S, Pinto B, Bonuccelli U, Bruschi F. Neurobiological studies on the relationship between toxoplasmosis and neuropsychiatric diseases. J Neurol Sci. 2015;351(1-2):3-8. doi:10.1016/j.jns.2015.02.028
- Layton J, Theiopoulou DC, Rutenberg D, et al. Clinical Spectrum, Radiological Findings, and Outcomes of Severe Toxoplasmosis in Immunocompetent Hosts: A Systematic Review. Pathogens. 2023;12(4):543. doi:10.3390/pathogens12040543
- Mustafa K, Hillyard J, Nowak E, et al. Toxoplasma myocarditis: An atypical case in an immunocompetent patient. IDCases. 2021;26:e01273. doi:10.1016/j.idcr.2021.e01273
- Kamal AM, Kamal AM, Abd El-Fatah AS, et al. Latent Toxoplasmosis is Associated with Depression and Suicidal Behavior. Arch Suicide Res. 2022;26(2):819-830. doi:10.1080/13811118.2020.1838368
- Latifi A, Flegr J. Beyond Latency: Chronic Toxoplasma Infection and Its Unveiled Behavioral and Clinical Manifestations-A 30-Year Research Perspective. Biomedicines. 2025;13(7):1731. doi:10.3390/biomedicines13071731
- Anand R, Jones CW, Ricks JH, et al. Acute primary toxoplasmosis in travelers returning from endemic countries. J Travel Med. 2012;19(1):57-60. doi:10.1111/j.1708-8305.2011.00564.x
- Elsheikha HM, Marra CM, Zhu XQ. Epidemiology, Pathophysiology, Diagnosis, and Management of Cerebral Toxoplasmosis. Clin Microbiol Rev. 2020;34(1):e00115-19. doi:10.1128/CMR.00115-19
- Garweg JG, Pleyer U. Treatment Strategy in Human Ocular Toxoplasmosis: Why Antibiotics Have Failed. J Clin Med. 2021;10(5):1090. doi:10.3390/jcm10051090
- Karnan A. Pulmonary toxoplasmosis in the immunocompetent. Pan Afr Med J. 2025;50:104. doi:10.11604/pamj.2025.50.104.47258
- Madireddy S, Mangat R. Toxoplasmosis. [Updated 2024 Oct 14]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK563286/
- Cerisola A, Francia M, Gesuele JP. Congenital toxoplasmosis. Semin Pediatr Neurol. 2025;54:101203. doi:10.1016/j.spen.2025.101203
- Flegr J. Thirty years of studying latent toxoplasmosis: behavioural, physiological, and health insights. Folia Parasitol (Praha). 2025;72:2025.005. doi:10.14411/fp.2025.005
- Pangracio E, de Macedo V, Alves PK. Pulmonary and Renal Toxoplasmosis in an Immunocompetent Adult Patient. J Med Cases. 2023;14(2):50-53. doi:10.14740/jmc4029
- CDC – DPDx – Toxoplasmosis. Centers for Disease Control and Prevention. May 5, 2025. Accessed September 22, 2025. https://www.cdc.gov/dpdx/toxoplasmosis/index.html.
- Toxoplasmosis. The Center for Food Security & Public Health – Iowa State University. January 2017. Accessed September 26, 2025. https://www.cfsph.iastate.edu/Factsheets/pdfs/toxoplasmosis.pdf.
- Pittman KJ, Knoll LJ. Long-Term Relationships: the Complicated Interplay between the Host and the Developmental Stages of Toxoplasma gondii during Acute and Chronic Infections. Microbiol Mol Biol Rev. 2015;79(4):387-401. doi:10.1128/MMBR.00027-15
- Toxoplasmosis: An important message for cat owners. U.S. Centers for Disease Control and Prevention. Accessed October 15, 2025. https://www.cdc.gov/parasites/toxoplasmosis/resources/printresources/catowners_2017.pdf.
- Latifi A, Flegr J, Kankova S. Re-assessing host manipulation in Toxoplasma: the underexplored role of sexual transmission – evidence, mechanisms, implications. Folia Parasitol (Praha). 2025;72:2025.015. doi:10.14411/fp.2025.015
- Tabares Tejada P, Cardona Maya WD. Toxoplasma gondii Infection in the Male Reproductive System: A Systematic Review. Acta Parasitol. 2025;70(1):29. doi:10.1007/s11686-024-00978-w
- Ben-Harari RR. Tick transmission of toxoplasmosis. Expert Rev Anti Infect Ther. 2019;17(11):911-917. doi:10.1080/14787210.2019.1682550
- Skotarczak BI. The role of ticks in transmission cycle of Toxoplasma gondii. Ann Parasitol. 2016;62(3):185–191. doi:10.17420/ap6203.52
- Zhou Y, Zhang H, Cao J, et al. Epidemiology of toxoplasmosis: role of the tick Haemaphysalis longicornis. Infect Dis Poverty. 2016;5:14. doi:10.1186/s40249-016-0106-0
- Dubey JP. Outbreaks of clinical toxoplasmosis in humans: five decades of personal experience, perspectives and lessons learned. Parasit Vectors. 2021;14(1):263. doi:10.1186/s13071-021-04769-4
- Toxoplasmosis: Causes, symptoms, diagnosis & treatment. Cleveland Clinic. September 8, 2025. Accessed September 22, 2025. https://my.clevelandclinic.org/health/diseases/9756-toxoplasmosis.
- About Infectious Mononucleosis (Mono). Centers for Disease Control and Prevention. May 9, 2024. Accessed September 22, 2025. https://www.cdc.gov/epstein-barr/about/mononucleosis.html.
- Akins GKH, Furtado JM, Smith JR. Diseases Caused by and Behaviors Associated with Toxoplasma gondii Infection. Pathogens. 2024;13(11):968. doi:10.3390/pathogens13110968
- Aksoy A, Tanir G, Ozkan M, et al. Acute disseminated encephalomyelitis associated with acute Toxoplasma gondii Infection. Pediatr Neurol. 2013;48(3):236-239. doi:10.1016/j.pediatrneurol.2012.11.004
- Lam AP, de Sordi D, Müller HHO, et al. Aggravation of symptom severity in adult attention-deficit/hyperactivity disorder by latent Toxoplasma gondii infection: a case-control study. Sci Rep. 2020;10(1):14382. doi:10.1038/s41598-020-71084-w
- Sutterland AL, Fond G, Kuin A, et al. Beyond the association. Toxoplasma gondii in schizophrenia, bipolar disorder, and addiction: systematic review and meta-analysis. Acta Psychiatr Scand. 2015;132(3):161-179. doi:10.1111/acps.12423
- Nayeri T, Sarvi S, Sharif M, Daryani A. Toxoplasma gondii: A possible etiologic agent for Alzheimer’s disease. Heliyon. 2021;7(6):e07151. doi:10.1016/j.heliyon.2021.e07151
- Wang J, Lin P, Li D, et al. Analysis of the Correlation Between Toxoplasma gondii Seropositivity and Alzheimer’s Disease. Pathogens. 2024;13(11):1021. doi:10.3390/pathogens13111021
- Kavakebian M, Ghandali T, Nasiri H, et al. The cognitive and neurological implications of toxoplasma gondii infection: Evidence from a systematic review and meta-analysis. Acta Trop. 2025;268:107705. doi:10.1016/j.actatropica.2025.107705
- Virus MA, Ehrhorn EG, Lui LM, Davis PH. Neurological and Neurobehavioral Disorders Associated with Toxoplasma gondii Infection in Humans. J Parasitol Res. 2021;2021:6634807. doi:10.1155/2021/6634807
- Del Grande C, Galli L, Schiavi E, et al. Is Toxoplasma gondii a Trigger of Bipolar Disorder?. Pathogens. 2017;6(1):3. doi:10.3390/pathogens6010003
- Haghbin M, Maani S, Bagherzadeh MA, et al. Latent Toxoplasmosis among Breast Cancer Patients in Jahrom, South of Iran. Int J Breast Cancer. 2023;2023:4792260. doi:10.1155/2023/4792260
- Cong W, Liu GH, Meng QF, et al. Toxoplasma gondii infection in cancer patients: prevalence, risk factors, genotypes and association with clinical diagnosis. Cancer Lett. 2015;359(2):307-313. doi:10.1016/j.canlet.2015.01.036
- Yu Y, Guo D, Qu T, et al. Increased Risk of Toxoplasma gondii Infection in Patients with Colorectal Cancer in Eastern China: Seroprevalence, Risk Factors, and a Case-Control Study. Biomed Res Int. 2020;2020:2539482. doi:10.1155/2020/2539482
- Wang Z, Qu T, Qi H, et al. Seroprevalence of Toxoplasma gondii infection in women with a gynecological tumor living in eastern China. PeerJ. 2022;10:e14569. doi:10.7717/peerj.14569
- Hodge JM, Coghill AE, Kim Y, et al. Toxoplasma gondii infection and the risk of adult glioma in two prospective studies. Int J Cancer. 2021;148(10):2449-2456. doi:10.1002/ijc.33443
- Abdollahi A, Razavian I, Razavian E, et al. Toxoplasma gondii infection/exposure and the risk of brain tumors: A systematic review and meta-analysis. Cancer Epidemiol. 2022;77:102119. doi:10.1016/j.canep.2022.102119
- Dupont D, Robert MG, Brenier-Pinchart MP,et al. Toxoplasma gondii, a plea for a thorough investigation of its oncogenic potential. Heliyon. 2023;9(11):e22147. doi:10.1016/j.heliyon.2023.e22147
- Hamouda MM, El-Saied AS, Zaher A, et al. Toxoplasma gondii: Seroprevalence and association with childhood brain tumors in Egypt. Acta Trop. 2024;251:107123. doi:10.1016/j.actatropica.2024.107123
- Bajnok J, Tarabulsi M, Carlin H, et al. High frequency of infection of lung cancer patients with the parasite Toxoplasma gondii. ERJ Open Res. 2019;5(2):00143-2018. doi:10.1183/23120541.00143-2018
- Chow E, Troy SB. The differential diagnosis of hypoglycorrhachia in adult patients. Am J Med Sci. 2014;348(3):186-190. doi:10.1097/MAJ.0000000000000217
- Geetha R, Tripathy K. Chorioretinitis. [Updated 2023 Aug 25]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK551705/
- Kimberlin DW, Banerjee R, Barnett E, Lynfield R, Sawyer MH. Toxoplasma gondii Infections (Toxoplasmosis) | Red Book: 2024–2027. American Academy of Pediatrics. April 2024. Accessed October 15, 2025. https://publications.aap.org/redbook/book/755/chapter/14082846/Toxoplasma-gondii-Infections294-Toxoplasmosis.
- Huang J, Zheng J, Liu B, et al. The association between Toxoplasma infection and mortality: the NHANES epidemiologic follow-up study. Parasit Vectors. 2022;15(1):284. doi:10.1186/s13071-022-05398-1
- Mihu AG, Olariu AT, Piros LE, et al. Toxoplasma gondii Seroprevalence and Associated Risk Factors in Psychiatric Patients Diagnosed with Moderate and Major Depression from Western Romania: A Case-Control Retrospective Study. Life (Basel). 2025;15(8):1157. doi:10.3390/life15081157
- Tan S, Tong WH, Vyas A. Impact of Plant-Based Foods and Nutraceuticals on Toxoplasma gondii Cysts: Nutritional Therapy as a Viable Approach for Managing Chronic Brain Toxoplasmosis. Front Nutr. 2022;9:827286. doi:10.3389/fnut.2022.827286
- Glover M, Tang Z, Sealock R, Jain S. Diarrhea as a Presenting Symptom of Disseminated Toxoplasmosis. Case Rep Gastrointest Med. 2017;2017:3491087. doi:10.1155/2017/3491087
- de Souza Giassi K, Costa AN, Apanavicius A, et al. Tomographic findings of acute pulmonary toxoplasmosis in immunocompetent patients. BMC Pulm Med. 2014;14:185. doi:10.1186/1471-2466-14-185
- Daher D, Shaghlil A, Sobh E, et al. Comprehensive Overview of Toxoplasma gondii-Induced and Associated Diseases. Pathogens. 2021;10(11):1351. doi:10.3390/pathogens10111351
- Satostegui M, Altcheh J, Moroni S, Moscatelli G. Guillain-Barré Syndrome Associated With Acute Toxoplasmosis in a 3-Year-old Boy. Pediatr Infect Dis J. 2022;41(8):e329-e331. doi:10.1097/INF.0000000000003550
- Tiwari I, Rolland CF, Popple AW. Cholestatic jaundice due to toxoplasma hepatitis. Postgrad Med J. 1982;58(679):299-300. doi:10.1136/pgmj.58.679.299
- Tian AL, Li GX, Elsheikha HM, et al. Seroepidemiology of Toxoplasma gondii infection in patients with liver disease in eastern China. Epidemiol Infect. 2017;145(11):2296-2302. doi:10.1017/S0950268817001327
- Schoukry NA, Farrag SA, Makarem SS, et al. Toxoplasma gondii in acute and chronic hepatitis. J Egypt Soc Parasitol. 1986;16(2):531-539.
- He JJ, Ma J, Elsheikha HM, et al. Transcriptomic analysis of mouse liver reveals a potential hepato-enteric pathogenic mechanism in acute Toxoplasma gondii infection. Parasit Vectors. 2016;9(1):427. doi:10.1186/s13071-016-1716-x
- Alvarado-Esquivel C, Estrada-Martínez S, Pérez-Álamos AR, et al. Toxoplasma gondii infection and insomnia: A case control seroprevalence study. PLoS One. 2022;17(6):e0266214. doi:10.1371/journal.pone.0266214
- Dupont D, Lin JS, Peyron F,et al. Chronic Toxoplasma gondii infection and sleep-wake alterations in mice. CNS Neurosci Ther. 2021;27(8):895-907. doi:10.1111/cns.13650
- Faraz M, Rosado FGN. Reactive Lymphadenopathies. Clin Lab Med. 2021;41(3):433-451. doi:10.1016/j.cll.2021.04.001
- de Haan L, Sutterland AL, Schotborgh JV, et al. Association of Toxoplasma gondii Seropositivity With Cognitive Function in Healthy People: A Systematic Review and Meta-analysis. JAMA Psychiatry. 2021;78(10):1103-1112. doi:10.1001/jamapsychiatry.2021.1590
- Kalantari N, Gorgani-Firouzjaee T, Moulana Z, et al. Toxoplasma gondii infection and spontaneous abortion: A systematic review and meta-analysis. Microb Pathog. 2021;158:105070. doi:10.1016/j.micpath.2021.105070
- Sorkhi H, Mollalo A, Bijani A, et al. Association between Toxoplasma gondii Infection and Nephrotic Syndrome Risk in Children: A Case-Control Study and Systematic Review. J Trop Pediatr. 2022;68(5):fmac067. doi:10.1093/tropej/fmac067
- Reeves GM, Mazaheri S, Snitker S, et al. A Positive Association between gondii Seropositivity and Obesity. Front Public Health. 2013;1:73. doi:10.3389/fpubh.2013.00073
- Alvarado-Esquivel C, Sánchez-Anguiano LF, Hernández-Tinoco J, et al. Influence of Toxoplasma Gondii Infection on Symptoms and Signs of Premenstrual Syndrome: A Cross-sectional Study. Eur J Microbiol Immunol (Bp). 2016;6(4):298-305. doi:10.1556/1886.2016.00030
- Molan AL, Ismail MH. Is there a positive association between Toxoplasma gondii seropositivity and obesity in diabetic patients?. Ann Parasitol. 2021;67(3):537-542. doi:10.17420/ap6703.368
- Januszewski J, Forma A, Kłodnicka K, et al. Microbiological bases of obsessive‑compulsive disorder – the role of viruses, bacteria, and parasites in the onset and progression of OCD. Acta Neurobiol Exp (Wars). 2024;84(3):230-242. doi:10.55782/ane-2024-2516
- Martínez-Girón R, Esteban JG, Ribas A, Doganci L. Protozoa in respiratory pathology: a review. Eur Respir J. 2008;32(5):1354-1370. doi:10.1183/09031936.00022008
- Aboukamar WA, Habib S, Tharwat S, et al. Association between toxoplasmosis and autoimmune rheumatic diseases in Egyptian patients. Reumatol Clin (Engl Ed). 2023;19(9):488-494. doi:10.1016/j.reumae.2023.03.006
- Hezarjaribi HZ, Azadeh H, Niksolat F, et al. Toxoplasma gondii infection in patients with rheumatoid arthritis and systemic lupus erythematous diseases: serological and molecular evidence. Ann Parasitol. 2021;67(2):223-228. doi:10.17420/ap6702.332
- Fischer S, Agmon-Levin N, Shapira Y, et al. Toxoplasma gondii: bystander or cofactor in rheumatoid arthritis. Immunol Res. 2013;56(2-3):287-292. doi:10.1007/s12026-013-8402-2
- Beil K. Toxoplasma gondii: A mind/body parasite? Naturopathic Doctor News & Review. December 21, 2007. Accessed September 26, 2025. https://ndnr.com/womens-health/toxoplasma-gondii-a-mindbody-parasite/.
- Postolache TT. Toxoplasma gondii and suicidal behaviour: discovery, cross-diagnostic confirmation and pathway exploration. Folia Parasitol (Praha). 2025;72:2025.025. doi:10.14411/fp.2025.025
- Zerekidze A, Li M, Refisch A, et al. Impact of Toxoplasma gondii and Human Microbiome on Suicidal Behavior: A Systematic Review. J Clin Med. 2024;13(2):593. doi:10.3390/jcm13020593
- Sutterland AL, Kuin A, Kuiper B, et al. Driving us mad: the association of Toxoplasma gondii with suicide attempts and traffic accidents – a systematic review and meta-analysis. Psychol Med. 2019;49(10):1608-1623. doi:10.1017/S0033291719000813
- Ranjan S, Panda AK. Seroprevalence of Toxoplasma gondii immunoglobulins and its association with systemic lupus erythematosus: A systematic review and meta-analysis. Lupus. 2024;33(11):1212-1219. doi:10.1177/09612033241273048
- Bassett P, Zabriskie BN, Catchpole A, Hedges D. Association between Toxoplasma gondii and systemic lupus erythematosus: A systematic review and meta-analysis. J Transl Autoimmun. 2022;5:100163. doi:10.1016/j.jtauto.2022.100163
- Cuan-Baltazar Y, Soto-Vega E. Microorganisms associated to thyroid autoimmunity. Autoimmun Rev. 2020;19(9):102614. doi:10.1016/j.autrev.2020.102614
- Galván-Ramírez ML, Soto-Hernández EE, Bojórquez-Pérez R. Coinfection of Toxoplasma gondii and Other Microorganisms: A Systematic Review and Meta-Analysis. Microorganisms. 2024;12(10):2106. doi:10.3390/microorganisms12102106
- Gouda MA, Saied SA, Edrees A, et al. Effect of concurrent infection of Helicobacter pylori with Toxoplasma gondii infection on gastric pathology. BMC Infect Dis. 2024;24(1):408. doi:10.1186/s12879-024-09270-8
- Mohammed K Sr. The Possible Association Between Chronic Toxoplasmosis and Type-2 Diabetes Mellitus In Women: A Case-Control Study. Cureus. 2025;17(2):e79120. doi:10.7759/cureus.79120
- Catchpole A, Zabriskie BN, Embley B, et al. Association between type-2 diabetes and Toxoplasma gondii seropositivity: a systematic review and meta-analysis. Folia Parasitol (Praha). 2025;72:2025.024. doi:10.14411/fp.2025.024
- Catchpole A, Zabriskie BN, Bassett P, et al. Association between Toxoplasma gondii Infection and Type-1 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Int J Environ Res Public Health. 2023;20(5):4436. doi:10.3390/ijerph20054436
- Gulshan JE, Lira SS, Qusar MMAS, et al. Association Between Toxoplasma gondii Infection and Serum Neurotransmitter Levels in Major Depressive Disorder Patients: A Case-Control Study in Bangladesh. J Parasitol Res. 2024;2024:7054920. doi:10.1155/japr/7054920
- Stanley Medical Research Institute: Neurotransmitters and gondii. Accessed October 15, 2025. https://stanleyresearch.org/patient-and-provider-resources/toxoplasmosis-schizophrenia-research/neurotransmitters-and-t-gondii/.
- Mohammed A, Ahmed M, Ibrahim N. The global seroprevalence of Toxoplasma gondii infection in workers occupationally exposed to animals (1972-2023): a systematic review and meta-analysis. Vet Q. 2024;44(1):1-18. doi:10.1080/01652176.2024.2396577
- Nayeri T, Sarvi S, Daryani A. Saliva and tear as useful tools for the diagnosis of Toxoplasma gondii in human specimens: a systematic review. Ann Parasitol. 2022;68(2):201-213. doi:10.17420/ap6802.426
- Loyola AM, Durighetto AF Jr, Silva DA, Mineo JR. Anti-Toxoplasma gondii immunoglobulins A and G in human saliva and serum. J Oral Pathol Med. 1997;26(4):187-191. doi:10.1111/j.1600-0714.1997.tb00456.x
- Cañedo-Solares I, Gómez-Chávez F, Luna-Pastén H, et al. What do anti-Toxoplasma gondii IgA and IgG subclasses in human saliva indicate?. Parasite Immunol. 2018;40(5):e12526. doi:10.1111/pim.12526
- Olariu TR, Blackburn BG, Press C, et al. Role of Toxoplasma IgA as Part of a Reference Panel for the Diagnosis of Acute Toxoplasmosis during Pregnancy. J Clin Microbiol. 2019;57(2):e01357-18. doi:10.1128/JCM.01357-18
- Tork M, Sadeghi M, Asgarian-Omran H, et al. Assessment of simultaneous IgM, IgG avidity, and IgA testing in diagnosis of acute toxoplasmosis in pregnant women: a systematic review and meta-analysis study. BMC Pregnancy Childbirth. 2025;25(1):537. doi:10.1186/s12884-025-07580-6
- Sensini A. Toxoplasma gondii infection in pregnancy: opportunities and pitfalls of serological diagnosis. Clin Microbiol Infect. 2006;12(6):504-512. doi:10.1111/j.1469-0691.2006.01444.x
- Paul M. [Use of the ISAGA method in detection of specific IgM, IgA, IgE antibodies in acquired and congenital toxoplasmosis]. Wiad Parazytol. 1997;43(1):39-51.
- Sobieszczańska B, Grzybek-Hryncewicz K, Rudzka A. [Detection of IgA antibodies as important markers of acute primary infection with Toxoplasma gondii]. Pol Merkur Lekarski. 1997;3(17):228-230.
- Mangiavacchi BM, Vieira FP, Bahia-Oliveira LM, Hill D. Salivary IgA against sporozoite-specific embryogenesis-related protein (TgERP) in the study of horizontally transmitted toxoplasmosis via gondii oocysts in endemic settings. Epidemiol Infect. 2016;144(12):2568-2577. doi:10.1017/S0950268816000960
- Garweg JG, de Groot-Mijnes JD, Montoya JG. Diagnostic approach to ocular toxoplasmosis. Ocul Immunol Inflamm. 2011;19(4):255-261. doi:10.3109/09273948.2011.595872
- Toxoplasma (Food Safety for Moms-to-be) | FDA. U.S. Food & Drug Administration. September 27, 2018. Accessed October 28, 2025. https://www.fda.gov/food/people-risk-foodborne-illness/toxoplasma-food-safety-moms-be.
- Toxoplasmosis. Mayo Clinic. November 3, 2022. Accessed October 20, 2025. https://www.mayoclinic.org/diseases-conditions/toxoplasmosis/symptoms-causes/syc-20356249.
- Lu YN, Lu JM, Jin GN, et al. A novel mechanism of resveratrol alleviates Toxoplasma gondii infection-induced pulmonary inflammation via inhibiting inflammasome activation. Phytomedicine. 2024;131:155765. doi:10.1016/j.phymed.2024.155765
- Lu YN, Shen XY, Lu JM, et al. Resveratrol inhibits Toxoplasma gondii-induced lung injury, inflammatory cascade and evidences of its mechanism of action. Phytomedicine. 2023;108:154522. doi:10.1016/j.phymed.2022.154522
- Lu JM, Jin GN, Lu YN, et al. Resveratrol modulates Toxoplasma gondii infection induced liver injury by intervening in the HMGB1/TLR4/NF-κB signaling pathway. Eur J Pharmacol. 2021;910:174497. doi:10.1016/j.ejphar.2021.174497
- Ghasemzadeh Rahbardar M, Sahebkar A. Resveratrol as a naturally occurring inflammasome modulator: Implications for health and disease. Iran J Basic Med Sci. 2025;28(10):1301-1318. doi:10.22038/ijbms.2025.87366.18879
- Avunduk AM, Avunduk MC, Baltaci AK, Moğulkoç R. Effect of melatonin and zinc on the immune response in experimental Toxoplasma retinochoroiditis. Ophthalmologica. 2007;221(6):421-425. doi:10.1159/000107504
- Machado NI, Dos Santos TAT, de Souza W, et al. Treatment with melatonin induces a reduction of Toxoplasma gondii development in LLC-MK2 cells. Parasitol Res. 2020;119(8):2703-2711. doi:10.1007/s00436-020-06766-5
- Liu S, Yan Z, Peng Y, et al. Lentinan has a beneficial effect on cognitive deficits induced by chronic Toxoplasma gondii infection in mice. Parasit Vectors. 2023;16(1):454. doi:10.1186/s13071-023-06023-5


