
Hippocrates, the Greek physician regarded as the “Father of Medicine,” is well known for his proclamation that “all disease begins in the gut” over two thousand years ago.1 Now, in 2023, we know his astute instinct was likely correct as we continue to learn how the gut microbiome and the numerous compounds it produces play a critical role in the prevention of many chronic diseases.
The substances produced by the microbiome are known as postbiotics. And, when the microbiome is unhealthy or in a state of “dysbiosis,” a postbiotic deficiency or imbalance could manifest and potentially lead to chronic disease, including asthma. In other words, according to the latest research, postbiotic compounds appear to be highly beneficial and essential for optimal health.2
To understand the concept of postbiotics, a brief review of prebiotics and probiotics is helpful. Probiotics are defined as “live microorganisms which, when administered in adequate amounts, confer a health benefit on the host.” However, probiotics may or may not produce the optimal beneficial postbiotic compounds in an adequate quantity, especially when consumption of prebiotics is not sufficient. A prebiotic is “a substrate that is selectively utilized by host microorganisms conferring a health benefit.”2,3
To produce postbiotics, the living probiotics, or gut bacteria, must digest and transform the prebiotics, such as dietary fiber and other substances naturally found in food, into postbiotics.2,3
In 2021, the International Scientific Association of Probiotics and Prebiotics (ISAPP) proposed the following definition for a postbiotic – a “preparation of inanimate microorganisms and/or their components that confers a health benefit on the host.” Essentially, postbiotics may contain the inactivated probiotics themselves and the compounds secreted by the probiotics while alive.4
Postbiotic substances include enzymes, bioactive peptides, short-chain fatty acids, antimicrobial peptides, polysaccharides, cell surface proteins, vitamins, plasmalogens, organic acids, and other compounds.2 Since the production of beneficial postbiotic compounds is not guaranteed, even with probiotic and prebiotic supplementation, researchers are now producing postbiotics in controlled environments outside the gut to assess their beneficial effects.
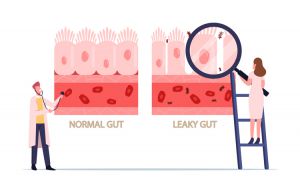
One way to assess gut health is by measuring intestinal permeability. The human intestine is approximately eight to nine meters long and provides one of the largest contact surfaces between our body and the outside world. While there is some controversy regarding the exact measurement of the surface area of the gut, the intestinal mucosa, or intestinal wall, boasts a significantly larger surface area than our skin!1,5
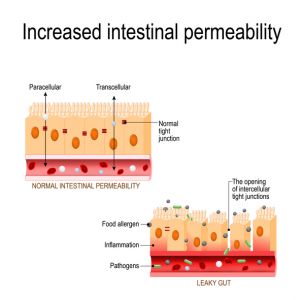
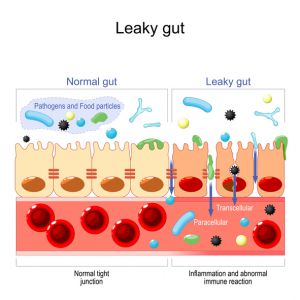
The intestinal mucosa is lined by tightly packed epithelial cells with intracellular tight junctions that form a barrier to regulate what crosses from the gut into the body.1 This barrier system must be selectively permeable to allow for the absorption of water and nutrients while continuously preventing potentially harmful substances from crossing into the body.6
In a healthy gut, the barrier remains strong and intact, but when the gut is not healthy, the barrier can be breached, which leads to inflammation. The inflammation can then further damage the intestinal epithelial cells and intracellular tight junctions. When the intracellular tight junctions do not function optimally, the intestinal mucosa becomes more permeable or “leaky,” leading to the vicious cycle of inflammation and dysfunction known as “leaky gut syndrome,” or increased intestinal permeability.
 What conditions are associated with a leaky gut?
What conditions are associated with a leaky gut?
- ADHD1
- Aging1
- Ankylosing spondylitis1
- Asthma7
- Autism1
- Autoimmune Hepatitis8
- Celiac Disease1
- Chronic Fatigue Syndrome1
- Colitis1
- Gestational Diabetes1
- Glioma1
- Inflammatory Bowel Diseases1
- Insulin Resistance1
- Irritable Bowel Syndrome1
- Hyperlipidemia1
- HIV1
- Liver Cirrhosis9
- Major Depressive Disorder1
- Multiple Sclerosis1
- Non-alcoholic fatty liver disease1
- Non-celiac gluten sensitivity1
- Obesity1
- Schizophrenia1
- Sepsis1
- Type 1 Diabetes1
- Type 2 Diabetes1
 Is it possible to prevent or reverse leaky gut?
Is it possible to prevent or reverse leaky gut?
Yes, several healing options could prevent or improve a leaky gut! One healing option is supporting a healthy gut microbiome. We know dysbiosis, or an unhealthy gut microbiome, contributes to increased intestinal permeability; therefore, any intervention that improves the health of the gut microbiome and resolves dysbiosis could also theoretically restore the healthy function of the intestinal barrier.10
One mechanism by which the intestinal bacteria in the gut support the integrity of the gut lining is via the production of postbiotics. Some postbiotics are antimicrobial substances that inhibit the proliferation of pathogenic organisms, allowing beneficial bacteria to thrive and manifest a healthy microbiome.10 Bacteria in the gut also secrete postbiotic compounds that directly support the integrity of the epithelial barrier in the gut, as we highlight below.

Spotlight on Lactobacilli-Derived Postbiotics
The Lactobacillus genus of probiotics includes some of the most widely studied microorganisms in the world. Given the important role that lactobacilli, also known as lactic acid bacteria, play in many biological processes, it is not surprising they receive abundant attention from the scientific community.11
Lactobacilli produce short-chain fatty acids (SCFAs), which are highly beneficial postbiotic compounds. SCFAs are made when dietary fiber is fermented by lactobacilli and other beneficial probiotic bacteria. SCFAs are the primary energy source for many epithelial cells, and a wealth of evidence indicates that SCFAs induce a “tightening” effect on tight junction permeability.12
Researchers obtain SCFAs and other postbiotic compounds from a bacterial culture by extracting the “cell-free culture supernatant,” which is the fluid that surrounds the living probiotic cells. Once the supernatant is extracted, the effects of the supernatant itself can be studied, or specific substances can be isolated from the supernatant for further study.13,14
The specific postbiotics secreted by lactobacilli can vary by species. For example, one of the first postbiotic compounds described with potent anti-pathogenic activity was reuterin, produced by Lactobacillus reuteri.15 Reuterin inhibits the growth of many gut pathogens, including Salmonella, Shigella, Proteus, Pseudomonas, Staphylococcus, fungi, and protozoa. As such, reuterin plays an important role in the maintenance of a healthy gut microbiome, thereby supporting optimal intestinal permeability.15
Similarly, lactobacilli-secreted supernatants, in general, appear to be a rich source of antibacterial postbiotics known as bacteriocins, which also restrict the growth and activities of specific pathogens to promote a healthy gut.15

Research has shown that lactobacilli-derived postbiotics strengthen intestinal permeability via several additional mechanisms.15 For example, the supernatant extracted from a Lactobacillus rhamnosus GG culture reverses alcohol-induced intestinal permeability by restoring optimal levels of tight junction proteins and the factors involved in mucin production.15
The Lactobacillus rhamnosus GG supernatant includes two specific postbiotic compounds, HM0539 and p40, known to stimulate the intestinal epithelial cells to secrete mucins, which are beneficial.14,15 The mucins create a dense mucus layer to block harmful substances from crossing the epithelial barrier, thereby improving intestinal permeability.14
Other postbiotics in lactobacilli-derived supernatants inhibit the death of the epithelial cells in the gut and promote their growth. Postbiotics produced by Lactobacillus plantarum, for example, positively influence intestinal integrity by promoting goblet cell differentiation, and the goblet cells produce the mucus that protects the intestinal epithelial cells.16,17 Lactobacilli also appear to support tissue repair through the secretion of postbiotics. Research has shown that lactobacilli-derived postbiotics known as indoles assist during epithelial damage to promote the healing of the gut lining.16,18
Lactate, another postbiotic produced by lactobacilli, supports the healing of the intestinal mucosa in several ways. According to animal studies, lactate promotes the healthy migration rate of intestinal epithelial cells by enhancing mitochondrial ATP production. Lactate also ameliorates colitis in animal studies by inducing the expression of Cdc42 and Pak1, two factors associated with healthy intestinal epithelial cell migration. SCFAs also exert a positive effect on wound healing since they enhance epithelial proliferation and differentiation to repair damage to the intestinal wall.16
Postbiotics can also indirectly contribute to the process of wound healing in the gut. A specific postbiotic from Lactobacillus brevis enhances the integrity of the epithelial barrier by improving platelet accumulation at the site of the wound.16 And, when purified from the supernatant of Lactobacillus casei ss rhamnosus, the soluble postbiotic known as LCrS5-30 reduces inflammation in the gut by stimulating the apoptosis, or cell death, of human immune cells without harming the intestinal epithelial cells. So, postbiotics also offer anti-inflammatory benefits.15
Concrete evidence of the impressive effects of postbiotic compounds secreted by different probiotic strains is progressively increasing. In recent years there has been a significant upsurge in the volume of research initiated to better understand the underlying postbiotic mechanisms.15
Much of the available literature focuses on lactobacilli, which is why we highlighted lactobacilli-derived postbiotics in this article.15 The positive impacts of postbiotics on gastrointestinal health and immune system function are just beginning to be explored in clinical studies.
Even so, the research completed thus far confirms that postbiotics play a significant role in modulating intestinal homeostasis, supporting the integrity of the intestinal mucosa, and balancing the gut microbiome, thereby conferring intriguing benefits to our health.19 Future research will continue to shed light on how postbiotics play a role in our overall health to prevent and treat many chronic diseases.
To screen for the presence of inflammation in the GI tract, consider ordering a Comprehensive GI Health Panel with Calprotectin. An overview of our GI Health Panels is available HERE.
To place a test order, click here. As a reminder, DiagnosTechs can drop ship test kits directly to your patients. You may select this option at the top of the order form.
Please visit our Provider Tools page for further information about Calprotectin and GI health testing.
YOU MAY ALSO ENJOY
BOTANICALS TO SUPPORT A HEALTHY GUT MICROBIOME
Did you know that consuming unusual foods, eating on the go, sleep disruptions, emotional stress, schedule changes, traveling, and other factors could significantly affect the health of your gut microbiome? If you traveled or experienced increased stress during the holidays, your gut microbiome might need a tune-up for the New Year.
POSTBIOTICS – THE NATURAL ANTIVIRALS OF THE FUTURE?
Microbial medicine has significantly evolved in recent years, and we now know nourishing the microbiome is a potential treatment option and prevention strategy for many health concerns. Microbial communities are found in many areas within the human body, including the gut, skin, lungs, sinuses, mouth, and urinary tract. The gut microbiome and its metabolites, in particular, play a fundamental role in many bodily functions, including nutrient production, nutrient absorption, immune system regulation, and the defense against infections, including viral infections.
POSTBIOTICS FOR BIOFILM MANAGEMENT
Microorganisms rarely exist as single cells living next to each other. Instead, they accumulate along surfaces to form polymicrobial aggregates known as a film, sludge, plaque, scum, or a ‘biofilm.’ The biofilm matrix is the extracellular material produced by the organisms in which the microorganisms embed themselves. This biofilm matrix consists of extracellular polymeric substances (EPS), such as polysaccharides, extracellular DNA, proteins, lipids, and inorganic solids. This matrix protects the microorganisms lurking inside the biofilm by acting as a shield against medications, ultraviolet light, probiotics, and other substances and organisms.
CALPROTECTIN PLUS PANEL FOR MONITORING GI INFLAMMATION
Calprotectin is an abundant neutrophil protein, and its presence in the stool indicates neutrophilic infiltration into the gut lumen, which is associated with severe inflammation. Elevated fecal calprotectin levels are present in patients with both infectious and inflammatory conditions, including inflammatory bowel disease (IBD).
References:
- Fasano A. All disease begins in the (leaky) gut: role of zonulin-mediated gut permeability in the pathogenesis of some chronic inflammatory diseases. F1000Res. 2020;9:F1000 Faculty Rev-69. doi:10.12688/f1000research.20510.1
- The Gut Microbiome and Asthma. InterPlexus. https://interplexus.com/blogs/news/the-gut-microbiome-and-asthma. Published May 5, 2022. Accessed June 27, 2022.
- Gibson GR, Hutkins R, Sanders ME, et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol. 2017;14(8):491-502. doi:10.1038/nrgastro.2017.75
- Salminen S, Collado MC, Endo A, et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics [published correction appears in Nat Rev Gastroenterol Hepatol. 2021 Jun 15;:]. Nat Rev Gastroenterol Hepatol. 2021;18(9):649-667. doi:10.1038/s41575-021-00440-6
- Ananda Rao A, Johncy S. Tennis Courts in the Human Body: A Review of the Misleading Metaphor in Medical Literature. Cureus. 2022;14(1):e21474. doi:10.7759/cureus.21474
- Thoo L, Noti M, Krebs P. Keep calm: the intestinal barrier at the interface of peace and war. Cell Death Dis. 2019;10(11):849. doi:10.1038/s41419-019-2086-z
- Baioumy SA, Elgendy A, Ibrahim SM, et al. Association between serum zonulin level and severity of house dust mite allergic asthma. Allergy Asthma Clin Immunol. 2021;17(1):86. doi:10.1186/s13223-021-00586-7
- Lin R, Zhou L, Zhang J, et al. Abnormal intestinal permeability and microbiota in patients with autoimmune hepatitis. Int J Clin Exp Pathol. 2015;8(5):5153-5160.
- Nishimura N, Kaji K, Kitagawa K, et al. Intestinal Permeability Is a Mechanical Rheostat in the Pathogenesis of Liver Cirrhosis. Int J Mol Sci. 2021;22(13):6921. doi:10.3390/ijms22136921
- Kocot AM, Jarocka-Cyrta E, Drabińska N. Overview of the Importance of Biotics in Gut Barrier Integrity. Int J Mol Sci. 2022;23(5):2896. doi:10.3390/ijms23052896
- De Filippis F, Pasolli E, Ercolini D. The food-gut axis: lactic acid bacteria and their link to food, the gut microbiome and human health. FEMS Microbiol Rev. 2020;44(4):454-489. doi:10.1093/femsre/fuaa015
- Binienda A, Twardowska A, Makaro A, et al. Dietary Carbohydrates and Lipids in the Pathogenesis of Leaky Gut Syndrome: An Overview. Int J Mol Sci. 2020;21(21):8368. doi:10.3390/ijms21218368
- Moradi M, Molaei R, Guimarães JT. A review on preparation and chemical analysis of postbiotics from lactic acid bacteria. Enzyme Microb Technol. 2021;143:109722. doi:10.1016/j.enzmictec.2020.109722
- Gao J, Li Y, Wan Y, et al. A Novel Postbiotic From Lactobacillus rhamnosus GG With a Beneficial Effect on Intestinal Barrier Function. Front Microbiol. 2019;10:477. doi:10.3389/fmicb.2019.00477
- Cicenia A, Scirocco A, Carabotti M, et al. Postbiotic activities of lactobacilli-derived factors. J Clin Gastroenterol. 2014;48 Suppl 1:S18-22. doi:10.1097/MCG.0000000000000231
- Filidou E, Kolios G. Probiotics in Intestinal Mucosal Healing: A New Therapy or an Old Friend? Pharmaceuticals (Basel). 2021;14(11):1181. doi:10.3390/ph14111181
- Gustafsson JK, Davis JE, Rappai T, et al. Intestinal goblet cells sample and deliver lumenal antigens by regulated endocytic uptake and transcytosis. Elife. 2021;10:e67292. doi:10.7554/eLife.67292
- Li X, Zhang B, Hu Y, et al. New Insights Into Gut-Bacteria-Derived Indole and Its Derivatives in Intestinal and Liver Diseases. Front Pharmacol. 2021;12:769501. doi:10.3389/fphar.2021.769501
- Liu Y, Wang J, Wu C. Modulation of Gut Microbiota and Immune System by Probiotics, Pre-biotics, and Post-biotics. Front Nutr. 2022;8:634897. doi:10.3389/fnut.2021.634897

